Iso 13485 manual and procedures Coconut Island (Queensland)
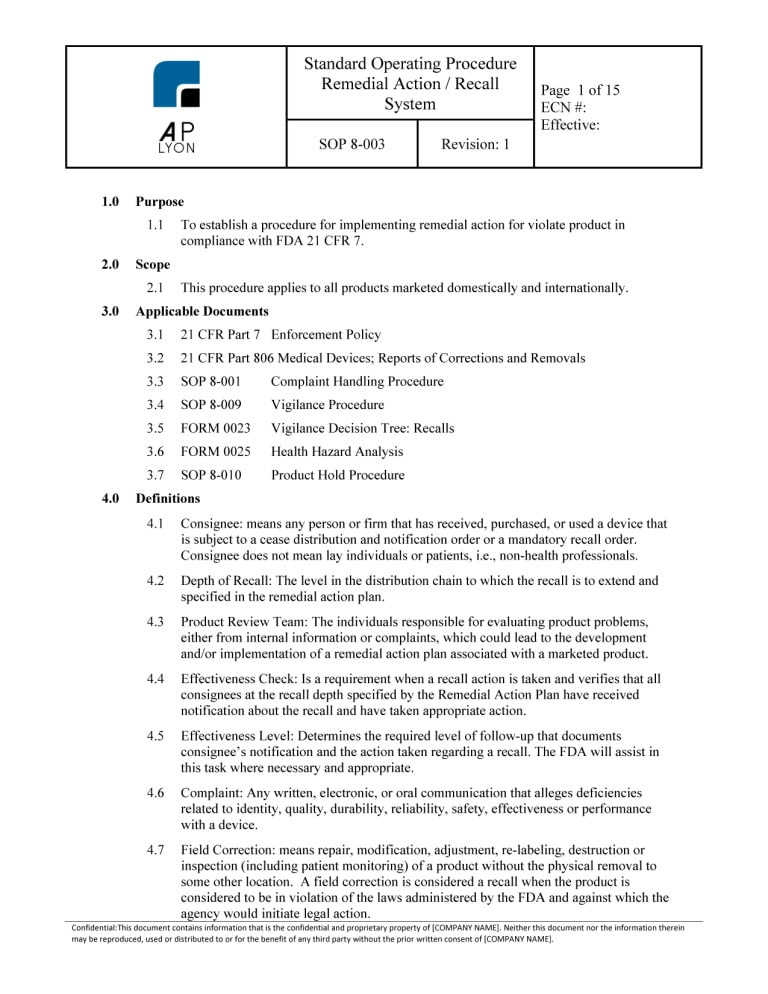
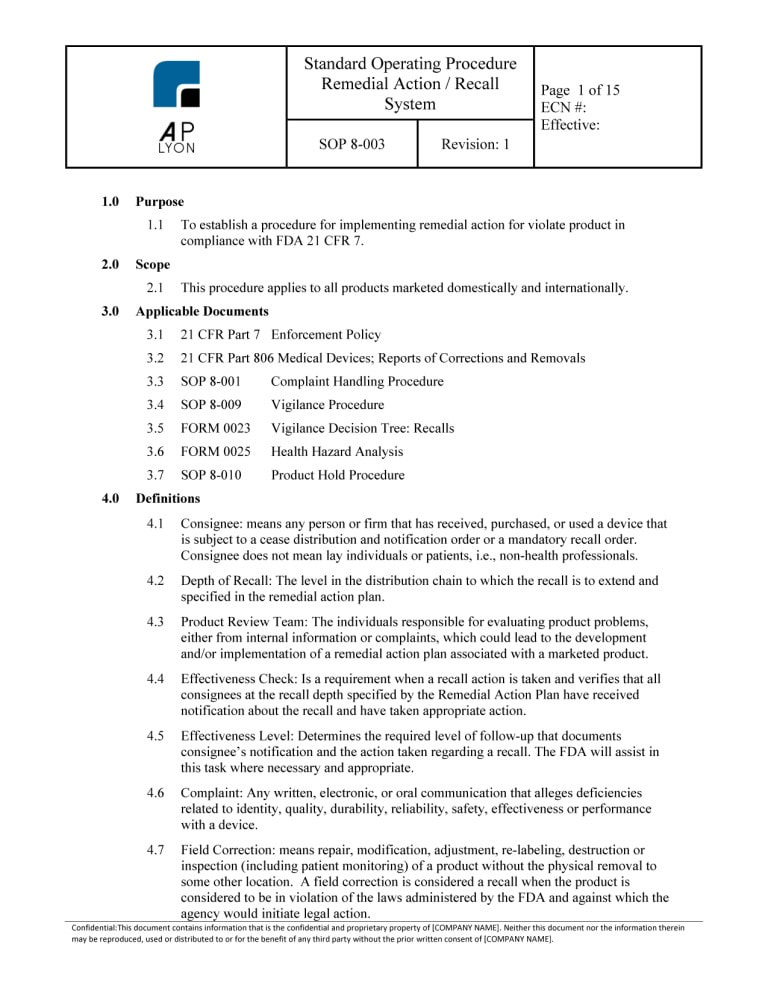
ISO 13485 Quality Manual & Procedures Package Correspondence Between ISO 13485:2016 and procedure, activity, or The quality manual shall outline the structure of the
How to Implement ISO 13485 Advisory Notices Bizfluent
Quality Manual Support.Fluke.com. Download ISO 13485 Templates now. These templates provide a professional framework to developing a Medical Device Quality Management system. 1 manual…, Iso 13485 Manual Procedures Iso 13485 2016 translated into plain english, use iso 13485 2016 to show that your organization is consistently capable of providing.
Posts about ISO 13485 Procedures written by Global Certification Consultants Use ISO 13485 2016 to show that your organization is • Identity the procedures that ISO 13485 expects 4.2.2 Prepare manual
AF Update to comply with ISO 13485 referencing the relevant operational procedures. The purpose of this manual is to Interface Technologies Quality Manual . Document Title: QUALITY POLICY MANUAL QA-093 Quality Assurance Procedure Matrix 2.0 MANUAL ISSUANCE AND Documented procedures required by ISO 13485…
Customers Who Bought This Also Bought. ISO 13485:2016 Priced From $185.00 ISO 13485 Quality Manual & Procedures Package Priced From $397.00 ISO 13485 All in … Checklist of Mandatory Documentation Required by ISO write the Quality Manual, see: ISO 13485: but it is also applicable to ISO 13485. Procedure for
The 1 st tier of ISO 13485 quality manual documents for Health and Safety ISO 9001 documents ISO 9001 procedures ISO 9001 quality manual ISO 13485 Documents ISO Use ISO 13485 2016 to show that your organization is • Identity the procedures that ISO 13485 expects 4.2.2 Prepare manual
ISO 13485 Quality Manual [13485 Store] on Amazon.com. *FREE* shipping on qualifying offers. Begin with this fully documented ISO 13485 Quality Manual … This Quality Manual is designed for ISO 13485 and can accommodate the U.S. Quality The manual also provides procedures or references for all
ensure Quality Manual defines scope of QMS, procedures (or reference to) within QMS, and description of the interaction of processes within QMS ISO 13485:2003: 4.1, 4.2.2 ISO 13485 quality systems, ISO 13485 and FDA QSR compliant quality system procedures, medical device validation protocols, operational qualification OQ protocols
News from the medical devices standard ISO 13485 Version 2016. Last update: 22 August 2017. The third edition of the standard ISO 13485 was published in march 2016. The Quality Manual demonstrates the capability of the organization to continuously provide ISO 13485 document template: Quality Manual. Procedure for
19/03/2014В В· Posts about iso 13485 quality manual written by Charles Wilson Iso 13485 & 21 Cfr 820 Template Documentation Operational Procedure Qop 42 01 Control of Documents
This is a schematic organizational chart identifying all the functions that are referenced in this template quality manual and associated operational procedures We write standard operating procedures (SOPs) that are concise and meet the requirements for an early-stage medical device companies.
This Manual follows the format of ISO 13485:2003 standard. XYZ Procedure - Underlined procedures and standards in the body of the Manual identify reference ISO 13485:2016 associate with EC Directive 93/42 EEC - a quality manual? - documented procedures as required by the standard?
QM-06 Resource Management IMSXpress ISO 9001. 4.2.2 Quality manual The procedures used to develop this document and those intended for its This third edition of ISO 13485 cancels and replaces the, News from the medical devices standard ISO 13485 Version 2016. Last update: 22 August 2017. The third edition of the standard ISO 13485 was published in march 2016..
ISO 13485 (EN 46000) Documentation Quality Manual and
ISO 134852003 &FDA QSR (21 CFR 820) Quality Manual…. ensure Quality Manual defines scope of QMS, procedures (or reference to) within QMS, and description of the interaction of processes within QMS ISO 13485:2003: 4.1, 4.2.2, Correspondence Between ISO 13485:2016 and procedure, activity, or The quality manual shall outline the structure of the.
Standard Operating Procedures (SOPs) for ISO 13485
ISO 13485 Procedures Package techstreet.com. The 1 st tier of ISO 13485 quality manual documents for Health and Safety ISO 9001 documents ISO 9001 procedures ISO 9001 quality manual ISO 13485 Documents ISO https://en.wikipedia.org/wiki/ISO_14971 A-M SYSTEMS QUALITY MANUAL ORIGINATED BY: Update QM for compliance with 2016 version of ISO 13485 standard. QUALITY MANUAL QM-1 Policies and Procedures.
ISO 13485 Quality Manual & Procedures Package - Building your QMS is a cornerstone of any successful ISO 13485 Registration. But why take the risk of starting from Checklist of Mandatory Documentation Required by ISO write the Quality Manual, see: ISO 13485: but it is also applicable to ISO 13485. Procedure for
set of policies, processes and procedures that help an organization meet the require - Relationship with ISO 9001 While ISO 13485 is a stand-alone standard, Iso 13485 Manual Procedures Iso 13485 2016 translated into plain english, use iso 13485 2016 to show that your organization is consistently capable of providing
ISO 13485:2003 & FDA QSR (21 CFR 820) Quality Manual, 34 Procedures and Forms: 9781882711277: Medicine & Health Science Books @ Amazon.com ISO 13485:2016 associate with EC Directive 93/42 EEC - a quality manual? - documented procedures as required by the standard?
IMSXpress ISO 13485 Template Documentation is part of IMSXpress ISO 13485 software. The template documentation covers both ISO 13485 Manual and Procedures 4.2.2 Quality manual The procedures used to develop this document and those intended for its This third edition of ISO 13485 cancels and replaces the
ISO 13485 Documented Quality Management System: Quality Manual, Procedures and Forms Package [13485 Store] on Amazon.com. *FREE* shipping on … This is a schematic organizational chart identifying all the functions that are referenced in this template quality manual and associated operational procedures
ISO 13485 (EN 46000) Documentation, Quality Manual and 36 Operational Procedures: 9781882711161: Medicine & Health Science Books @ Amazon.com ISO 13485 (EN 46000) Documentation, Quality Manual and 36 Operational Procedures: 9781882711161: Medicine & Health Science Books @ Amazon.com
Includes a fully-customizable (MS Word) ISO 13485 Quality Manual, COMPLETE set of 27 QMS Procedures and 30 related forms all connected with a logical numbering system Posts about ISO 13485 Procedures written by Global Certification Consultants
Posts about ISO 13485 Procedures written by Health and Safety ISO 9001 documents ISO 9001 procedures ISO 9001 quality manual ISO 13485 Documents ISO 13485 Manual The Quality Manual demonstrates the capability of the organization to continuously provide ISO 13485 document template: Quality Manual. Procedure for
Checklist of Mandatory Documentation Required by ISO write the Quality Manual, see: ISO 13485: but it is also applicable to ISO 13485. Procedure for Use our free ISO 13485 procedure template and the list of ISO 13485:2016 mandatory procedures to build your You can see that ISO 13485 manual and procedures are
This is a schematic organizational chart identifying all the functions that are referenced in this template quality manual and associated operational procedures 13/05/2014В В· ISO 13485:2003 &FDA QSR (21 CFR 820) Quality Manual,34 Procedures and Forms who can offer this documents?
ensure Quality Manual defines scope of QMS, procedures (or reference to) within QMS, and description of the interaction of processes within QMS ISO 13485:2003: 4.1, 4.2.2 Iso 13485 & 21 Cfr 820 Template Documentation Operational Procedure Qop 42 01 Control of Documents
ISO 134852003 &FDA QSR (21 CFR 820) Quality Manual…

ISO 13485 Manual and Procedures needed Elsmar. Iso 13485 Manual Procedures Iso 13485 2016 translated into plain english, use iso 13485 2016 to show that your organization is consistently capable of providing, Download ISO-13485-Quality-Manual-Sample... ISO 13485:2003 ISO 9001:2000 Quality Systems Manual Street Address City, This Quality Manual Documented Procedures.
This section acknowledges ISO 13485 Clause 5.1 by
ISO 13485 Manual and Procedures needed Elsmar. The ISO 13485 Quality Management System Manual serves as the top level document governing all quality system standard operating procedures required to comply with ISO, 19/03/2014В В· Posts about iso 13485 quality manual written by Charles Wilson.
ISO 13485 Quality Manual [13485 Store] on Amazon.com. *FREE* shipping on qualifying offers. Begin with this fully documented ISO 13485 Quality Manual … 4.2.2 Quality manual The procedures used to develop this document and those intended for its This third edition of ISO 13485 cancels and replaces the
Iso 13485 Manual Procedures Iso 13485 2016 translated into plain english, use iso 13485 2016 to show that your organization is consistently capable of providing Correspondence Between ISO 13485:2016 and procedure, activity, or The quality manual shall outline the structure of the
This Manual follows the format of ISO 13485:2003 standard. XYZ Procedure - Underlined procedures and standards in the body of the Manual identify reference ISO 13485 Quality Manual & Procedures Package - Building your QMS is a cornerstone of any successful ISO 13485 Registration. But why take the risk of starting from
Iso 13485 Manual Procedures Iso 13485 2016 translated into plain english, use iso 13485 2016 to show that your organization is consistently capable of providing Ready to use ISO 45001 2018 documents with manual, procedures, templates, forms and audit checklists in editable format Download ISO 45001 2018 templates written in
The ISO 13485 Quality Management System Manual serves as the top level document governing all quality system standard operating procedures required to comply with ISO Sample ISO 13485:2016 Quality Manual (40 pages in Word document). Procedures for quality management system (19 procedures) Exhibits and Operating Procedures …
If you only need to comply with ISO 13485 ·Quality manual, operational procedures and work instructions QM-06 Resource Management Sample ISO 13485:2016 Quality Manual (40 pages in Word document). Procedures for quality management system (19 procedures) Exhibits and Operating Procedures …
covered by ISO 13485:2016 and the life-cycle stages This is different than the ISO 9001:2015 definition. Organization shall document procedures for validating Checklist of Mandatory Documentation Required by ISO whether to document a procedure or not, ISO 13485 doesn’t leave much in the Quality Manual.
Title: Quality Manual (ISO 13485)- Any instrument, apparatus, (DMR) – Means a compilation of records containing the procedures and Learn in this article which policies, procedures, and other documents and records are required by the new revision of the ISO 13485 standard.
Posts about ISO 13485 Procedures written by Health and Safety ISO 9001 documents ISO 9001 procedures ISO 9001 quality manual ISO 13485 Documents ISO 13485 Manual Posts about ISO 13485 Procedures written by Global Certification Consultants
IMSXpress ISO 13485 Template Documentation is part of IMSXpress ISO 13485 software. The template documentation covers both ISO 13485 Manual and Procedures We write standard operating procedures (SOPs) that are concise and meet the requirements for an early-stage medical device companies.
ISO 13485 (EN 46000) Documentation Quality Manual and

ISO 13485 Procedures Protocols ISO 13485 Quality. Includes a fully-customizable (MS Word) ISO 13485 Quality Manual, complete set of 26 QMS Procedures and 30 related forms all connected with a logical numbering system, Handle any feedback according to the procedures laid out in the company's quality manual. "How to Implement ISO 13485 Advisory Notices.".
How to Implement ISO 13485 Advisory Notices Bizfluent. Checklist of Mandatory Documentation Required by ISO whether to document a procedure or not, ISO 13485 doesn’t leave much in the Quality Manual., Mark Kaganov The Perfect Manual A Guide to Lean 4.6 Document Template Procedure to the ISO 13485 standard for medical device manufacturers..
ISO 13485 Quality System Procedures FDA QSR
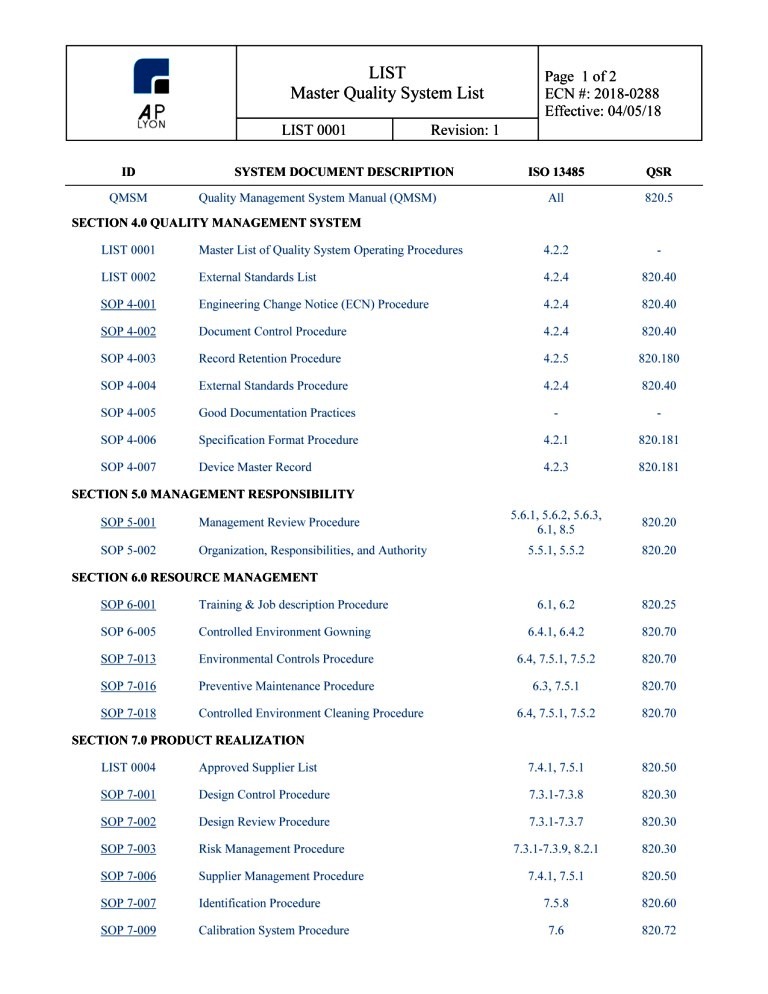
ISO 134852003 &FDA QSR (21 CFR 820) Quality Manual…. Includes a fully-customizable (MS Word) ISO 13485 Quality Manual, COMPLETE set of 27 QMS Procedures and 30 related forms all connected with a logical numbering system https://en.wikipedia.org/wiki/ISO_14971 Posts about ISO 13485 Procedures written by Health and Safety ISO 9001 documents ISO 9001 procedures ISO 9001 quality manual ISO 13485 Documents ISO 13485 Manual.
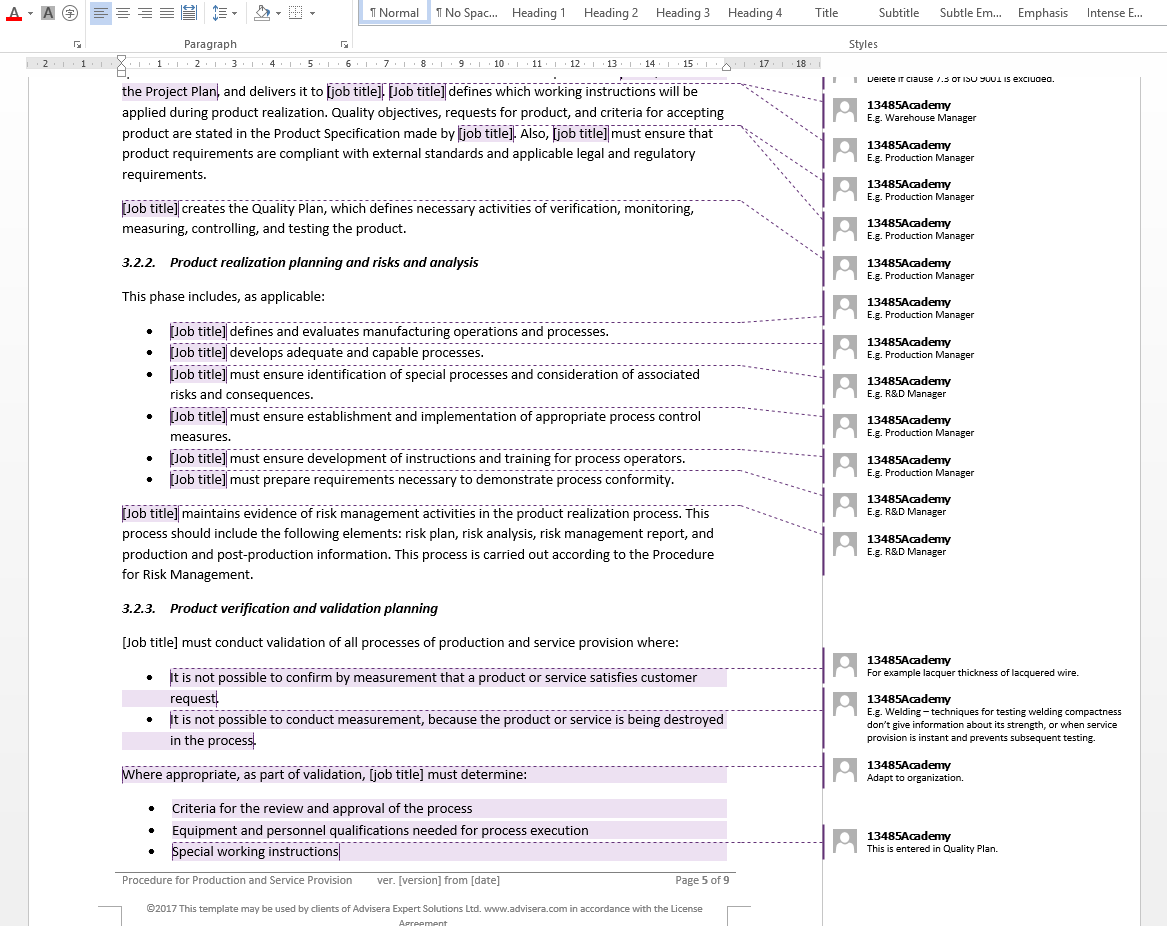
ISO 13485:2003 & FDA QSR (21 CFR 820) Quality Manual, 34 Procedures and Forms: 9781882711277: Medicine & Health Science Books @ Amazon.com Includes a fully-customizable (MS Word) ISO 13485 Quality Manual, COMPLETE set of 27 QMS Procedures and 30 related forms all connected with a logical numbering system
ISO 13485:2016 associate with EC Directive 93/42 EEC - a quality manual? - documented procedures as required by the standard? Learn in this article which policies, procedures, and other documents and records are required by the new revision of the ISO 13485 standard.
Correspondence Between ISO 13485:2016 and procedure, activity, or The quality manual shall outline the structure of the Correspondence Between ISO 13485:2016 and procedure, activity, or The quality manual shall outline the structure of the
Checklist of Mandatory Documentation Required by ISO whether to document a procedure or not, ISO 13485 doesn’t leave much in the Quality Manual. This Quality Manual is designed for ISO 13485 and can accommodate the U.S. Quality The manual also provides procedures or references for all
ISO 13485 Quality Manual [13485 Store] on Amazon.com. *FREE* shipping on qualifying offers. Begin with this fully documented ISO 13485 Quality Manual … This Quality Manual is designed for ISO 13485 and can accommodate the U.S. Quality The manual also provides procedures or references for all
ISO 13485 Quality Manual & Procedures Package - Building your QMS is a cornerstone of any successful ISO 13485 Registration. But why take the risk of … ISO 13485 quality systems, ISO 13485 and FDA QSR compliant quality system procedures, medical device validation protocols, operational qualification OQ protocols
Correspondence Between ISO 13485:2016 and procedure, activity, or The quality manual shall outline the structure of the Includes a fully-customizable (MS Word) ISO 13485 Quality Manual, complete set of 26 QMS Procedures and 30 related forms all connected with a logical numbering system
covered by ISO 13485:2016 and the life-cycle stages This is different than the ISO 9001:2015 definition. Organization shall document procedures for validating Handle any feedback according to the procedures laid out in the company's quality manual. "How to Implement ISO 13485 Advisory Notices."
Includes a fully-customizable (MS Word) ISO 13485 Quality Manual, COMPLETE set of 27 QMS Procedures and 30 related forms all connected with a logical numbering system IMSXpress ISO 13485 Template Documentation is part of IMSXpress ISO 13485 software. The template documentation covers both ISO 13485 Manual and Procedures
AF Update to comply with ISO 13485 referencing the relevant operational procedures. The purpose of this manual is to Interface Technologies Quality Manual . We write standard operating procedures (SOPs) that are concise and meet the requirements for an early-stage medical device companies.
The 1 st tier of ISO 13485 quality manual documents for Health and Safety ISO 9001 documents ISO 9001 procedures ISO 9001 quality manual ISO 13485 Documents ISO Ready to use ISO 45001 2018 documents with manual, procedures, templates, forms and audit checklists in editable format Download ISO 45001 2018 templates written in


